IRB Training
Required Education for Human Subjects Researchers
Individuals who will be involved in the design or conduct of human subjects research must fulfill the mandatory training requirements and have an approved IRB protocol before beginning any research involving human subjects. These individuals are considered to be "key personnel" and include Principal Investigator(s), all individuals responsible for the design or conduct of the study, and those individuals identified as key personnel of consortium participants or alternate performance sites if they are participating in research that involves human subjects.
Caltech's training program involves on-line training modules found on the Collaborative Institutional Training Initiative (CITI) website. CITI is available by logging into your access.caltech homepage, under the Research Services section, "Research Ethics Education (CITI)".
Training must be current within 3-5 years, depending upon the type of research performed. See "How Often is Refresher Training Required?" for more information. Researchers are no longer required to attach a copy of IRB Completion Certificates to their IRB protocols.
Researchers must complete one of the three below learner group training modules based on role and the type of human subjects research conducted.
SOCIAL, BEHAVIORAL and EDUCATIONAL (SBE) RESEARCH
Social and behavioral research refers broadly to research that deals with human attitudes, beliefs, and behaviors and is often characterized by data collection methods such as questionnaires, interviews, focus groups, direct or participant observation, and non-invasive physical measurements
Most researchers in the Division of Humanities and Social Sciences will take this course grouping.
BIOMEDICAL (BIO) RESEARCH
Biomedical research refers to the study of human physiology and specific diseases and conditions (mental or physical), including detection, cause, prophylaxis, treatment and rehabilitation of persons; the design of methods, and devices used to diagnose, support and maintain the individual during and after treatment for specific diseases or conditions; and/or the scientific investigation required to understand the underlying life processes which affect disease and human well-being, including such areas as cellular and molecular bases of diseases, genetics, immunology.
Most researchers in the Divisions of Engineering and Applied Science, Chemistry and Chemical Engineering, and Biology and Biological Engineering will take this course grouping.
HYBRID RESEARCH
This training is available for researchers whose labs perform both Social & Behavioral and Biomedical research or for protocols that are social behavioral studies that involve the use of drugs or devices, radiation or radiolabeled tracers, and other invasive procedures (e.g., fMRI, electrical stimulation).
CLINICAL TRIALS
If your research includes clinical trials, you must also complete the Good Clinical Practices training module corresponding to your learner group.
- GCP - Social and Behavioral Research Best Practices for Clinical Research (for SBE researchers)
- GCP for Clinical Investigations of Devices (for BIO researchers)
ADDITIONAL TRAINING MODULES
Supplemental training modules may be required depending upon the research conducted. See "What Supplemental Modules Do I Need to Take?"
Following the completion of initial IRB training, researchers that work with human subjects are required to complete refresher training on a regular basis. This refresher training occurs on different time cycles, dependent upon the type of research involved. The IRB will inform you of the appropriate modules upon submission of a protocol.
Department of Defense = Every 3 YEARS
Research is considered to involve the DoD when the research if funded by a component of the DoD (e.g. Army, Navy, Air Force), when the research involves cooperation, collaboration, or other type of agreement with a component of DoD, when the research uses property, facilities, or assets of a component of DoD, or when the subject population will intentionally include personnel from a component of the DoD.
Clinical Trials = Every 3 YEARS
Effective January 2, 2017 - NIH expects all NIH-funded clinical investigators and clinical trial staff who are involved in the design, conduct, oversight, or management of clinical trials to be trained in Good Clinical Practice (GCP). The NIH defines clinical trials as a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
All Other Human Subjects Studies = Every 5 YEARS
Caltech's training program for human subjects researchers involves on-line training modules found on the Collaborative Institutional Training Initiative (CITI) website. CITI is available by logging into your access.caltech homepage, under the Research Services section, "Research Ethics Education (CITI)".
If this is your first time logging into CITI, you will be asked to log-in through your organization. Choose California Institute of Technology and follow the log-in prompts.

After logging in, choose the "View Courses" option for California Institute of Technology.
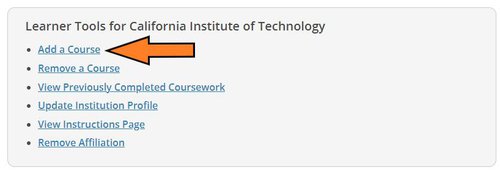
From the next page, scroll to the bottom and click on "Add a Course" in the Learner Tools box.
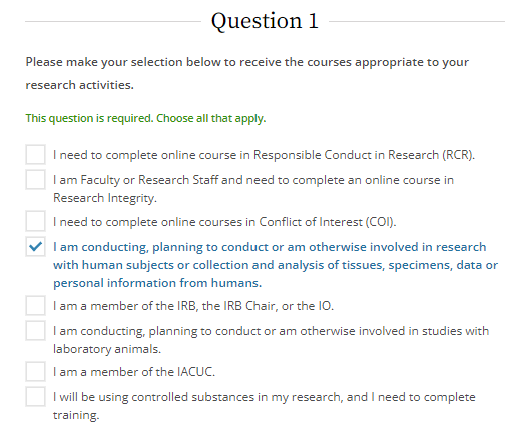
Select the options below based upon your research activities. If you are working with human subjects, you must choose the fourth option.
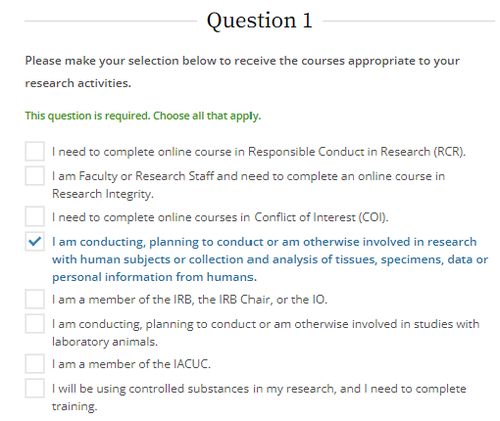
Choose one learner group based on role and the type of human subjects research conducted.

Choose any elective modules that may be related to your study.

You will be enrolled in the Basic Course and any additional Caltech specific modules that you choose.
If you have not chosen the necessary courses, you will be instructed by the IRB to complete additional supplemental modules based upon your research.
Your CITI training certificates can be found by choosing "My Records" from your CITI homepage.

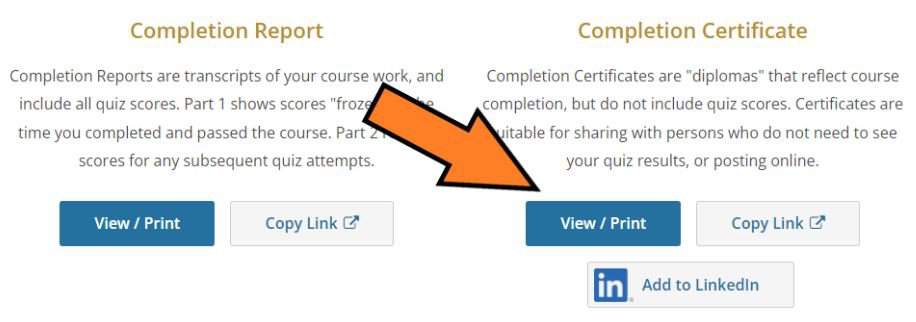
From the next screen, choose the appropriate course, and click "View-Print-Share".

From the next screen, choose "View/Print" under the Completion Certificate column.
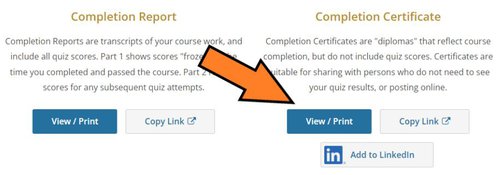
Save the pdf copy of the Certificate to your files. A copy of your Certificate NO LONGER needs to be attached to your IRB protocols.
An updated list of modules will be posted shortly.
Basic modules are assigned by CITI. The IRB may ask you to complete additional Supplemental modules (as required by your research).
The NIH Protecting Human Research Participants training option is no longer available from the NIH as of September 26, 2018. Please see the following announcement from the NIH:
https://grants.nih.gov/grants/guide/notice-files/NOT-OD-18-221.html
Certificates that are current within the parameters outlined above are valid.